Steam resistometer (BIER/CIER) Quality control and product development at the highest level
The Lautenschläger steam resistometer exceeds the requirements of
EN ISO 18472:2018-11 (Sterilization of health care products – Biological and chemical indicators – Test equipment). The BIER / CIER generates an almost ideal rectangular profile (square wave) for the pressure and temperature curve with extremely high accuracy.
The unsurpassed measuring and control accuracy of the Lautenschläger steam resistometer(BIER/CIER) reliably ensures reproducible results. For example, the turnover behavior of indicators can be tested precisely without rising and cooling times influencing the results.
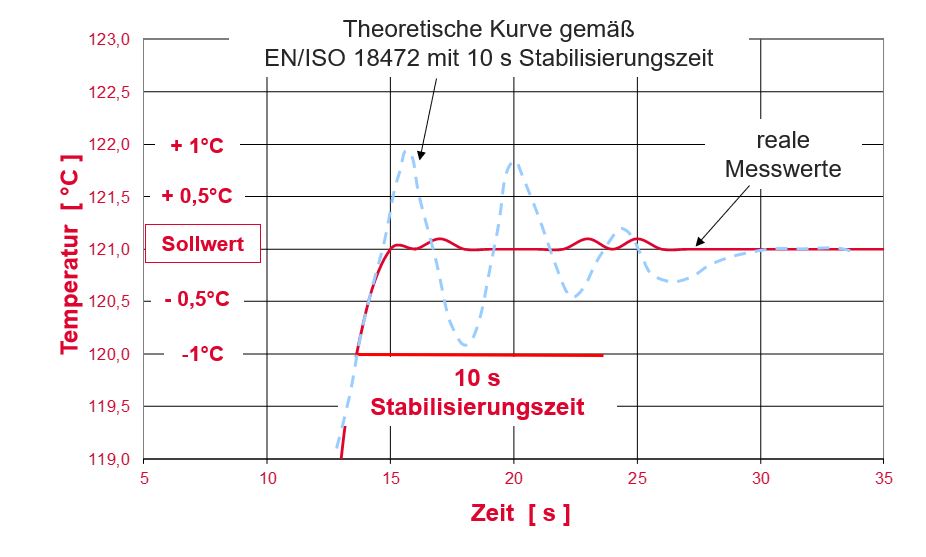
Comparison of the specifications from the standard (DIN EN ISO 18472) with real measured values of the Lautenschläger BIER / CIER
The Lautenschläger Steril software allows individual programming of sterilization cycles. Both exposure times (holding times) and temperatures can be freely programmed. Air injection cylinders, leakage valves, sight glasses and many other optional extras are available, allowing product development to be optimized and accelerated.



